
The effect of adding the ammonia is to lower this concentration still further. This is a reversible reaction, but the complex is very stable, and the position of equilibrium lies well to the right.Ī solution in contact with one of the silver halide precipitates will contain a very small concentration of dissolved silver ions. The ammonia combines with silver ions to produce a complex ion called the diamminesilver(I) ion, +. You can see that the compounds are all pretty insoluble, but become even less soluble as you go from the chloride to the bromide to the iodide. Note: These figures come from the Chemistry Data Book by Stark and Wallace. Solubility products only work with compounds which are very, very sparingly soluble.) (You can't quote a solubility product value for silver fluoride because it is too soluble. Look at the way the solubility products vary from silver chloride to silver iodide. Note: If your syllabus says that you need to know about solubility product calculations, you might be interested in my chemistry calculations book where they are explained in detail. Enough of the solid is precipitated so that the ionic product is lowered to the value of the solubility product. If the product of the concentrations would exceed this value, you do get a precipitate.Įssentially, the product of the ionic concentrations can never be greater than the solubility product value. If the actual concentrations of the ions in solution produce a value less than the solubility product, you don't get a precipitate. The square brackets have their normal meaning, showing concentrations in mol dm -3. For the silver halides, the solubility product is given by the expression: This value can be quoted as a solubility product. A precipitate will only form if the concentrations of the ions in solution in water exceed a certain value - different for every different compound. There is no such thing as an absolutely insoluble ionic compound. Precipitate is insoluble in ammonia solution of any concentration Precipitate is almost unchanged using dilute ammonia solution, but dissolves in concentrated ammonia solution to give a colourless solution Precipitate dissolves to give a colourless solution Silver fluoride is soluble, and so you don't get a precipitate.Ĭonfirming the precipitate using ammonia solutionĪmmonia solution is added to the precipitates. The precipitates are the insoluble silver halides - silver chloride, silver bromide or silver iodide. All the absence of a precipitate shows is that you haven't got chloride, bromide or iodide ions present.

The absence of a precipitate with fluoride ions doesn't prove anything unless you already know that you must have a halogen present and are simply trying to find out which one. You couldn't be sure which you had unless you compared them side-by-side.Īll of the precipitates change colour if they are exposed to light - taking on grey or purplish tints.
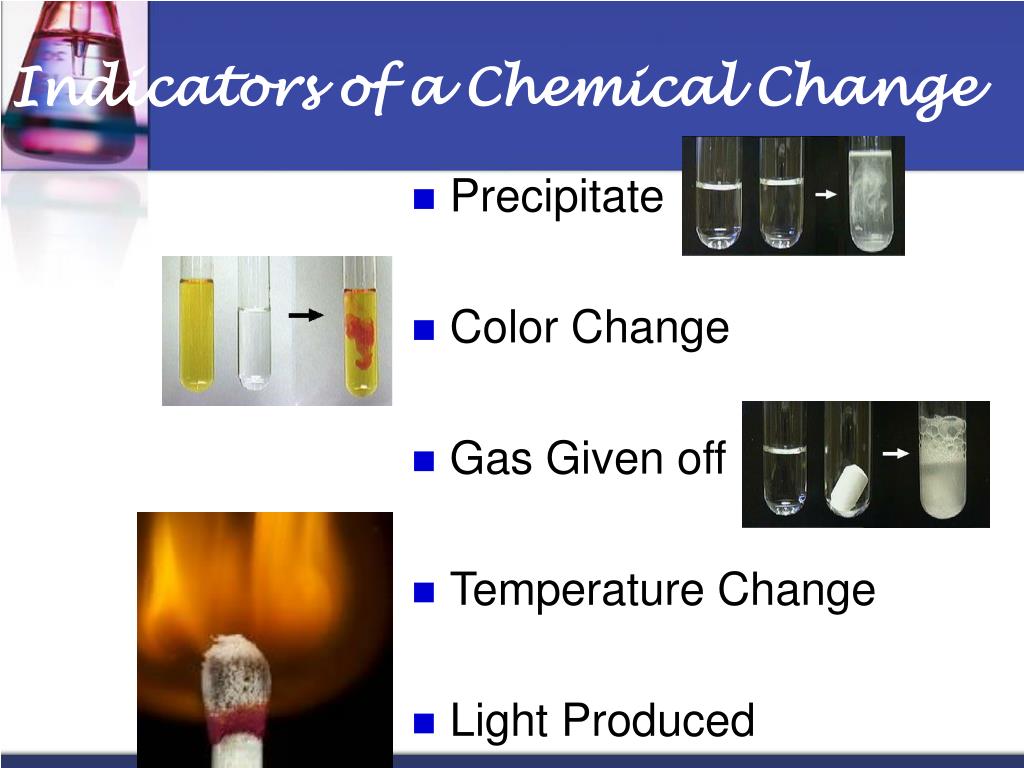
The chloride precipitate is obviously white, but the other two aren't really very different from each other.

The chloride, bromide and iodide precipitates are shown in the photograph: Silver nitrate solution is then added to give: ion present (Remember: silver nitrate + dilute nitric acid.) The nitric acid reacts with, and removes, other ions that might also give a confusing precipitate with silver nitrate. The solution is acidified by adding dilute nitric acid. If you start from a solid, it must first be dissolved in pure water. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution.


 0 kommentar(er)
0 kommentar(er)
